To view the Multimedia News Release, go to: https://www.multivu.com/players/English/8004555-amgen-aimovig-erenumab-phase3-strive-study-migraines/.
"STRIVE is the first fully reported Phase 3 study of the CGRP pathway monoclonal antibodies, and it clearly shows that blocking this pathway can reduce the impact of migraine," said
STRIVE enrolled 955 patients experiencing a mean baseline of 8.3 monthly migraine days, who were randomized to receive either placebo or subcutaneous Aimovig 140 mg or 70 mg once a month, for six months. Patients taking Aimovig at the higher dose experienced a significant 3.7-day reduction in monthly migraine days (3.2-day reduction with 70 mg, 1.8-day reduction with placebo; p<0.001 for both doses versus placebo). Fifty percent of patients taking Aimovig 140 mg had their migraine days cut by 50 percent or greater, representing a significantly higher likelihood of achieving this response compared to placebo (43.3 percent at 70 mg and 26.6 percent with placebo; odds ratios of 2.8 and 2.1 respectively for 140 mg and 70 mg; p<0.001 for both doses versus placebo). All STRIVE endpoints were assessed from baseline to the average of the last three months of the double-blind treatment phase of the study (months 4, 5, 6).
"There is a clear unmet need for efficacious, innovative therapies for the prevention of migraine. Publication of these data underscores the significance of the CGRP receptor blocker Aimovig as potentially the first available treatment targeting a pathophysiologically relevant pathway for one of the most common causes of disability across the globe," said
Other secondary endpoint results from the Phase 3 study include:
In the STRIVE study, the overall safety and tolerability profiles of Aimovig were similar to that of placebo. Adverse events occurring in greater than five percent of all treatment arms were nasopharyngitis and upper respiratory tract infection. More than 90 percent of patients in the Aimovig-treated arms completed the six-month study. Adverse events leading to discontinuation of treatment occurred in 2.2 percent of patients in either Aimovig treatment arm and in 2.5 percent of patients receiving placebo. STRIVE contributes to an extensive body of evidence in support of the efficacy, safety and tolerability profile of Aimovig, including four placebo-controlled Phase 2 and Phase 3 clinical studies involving more than 2,600 patients, as well as open-label extensions up to five years in duration.
Aimovig is the first and only investigational therapy targeting the CGRP pathway to have received
About STRIVE
STRIVE (Study to Evaluate the Efficacy and Safety of Erenumab in Migraine Prevention, NCT02456740) is a global Phase 3, multicenter, randomized 24-week, double-blind, placebo-controlled study evaluating the safety and efficacy of Aimovig in episodic migraine (characterized in this study as ≥4 to <15 migraine days per month and <15 headache days per month on average across the three months before screening) prevention. In the study, 955 patients were randomized to receive once-monthly subcutaneous placebo, or Aimovig (70 mg or 140 mg) in a 1:1:1 ratio. Patients experienced between four and 14 migraine days each month, with an average of 8.3 migraine days per month at baseline. The primary endpoint was change in mean monthly migraine days from baseline over the last three months of the double-blind treatment phase of the study (months 4, 5 and 6). Secondary study endpoints assessed at six months included reduction of at least 50 percent from baseline in mean monthly migraine days, change from baseline in mean monthly acute migraine-specific medication days, and reductions from baseline in both mean impact on everyday activities domain and mean physical impairment domain scores on the Migraine Physical Function Impact Diary (MPFID).
About Aimovig™ (erenumab)
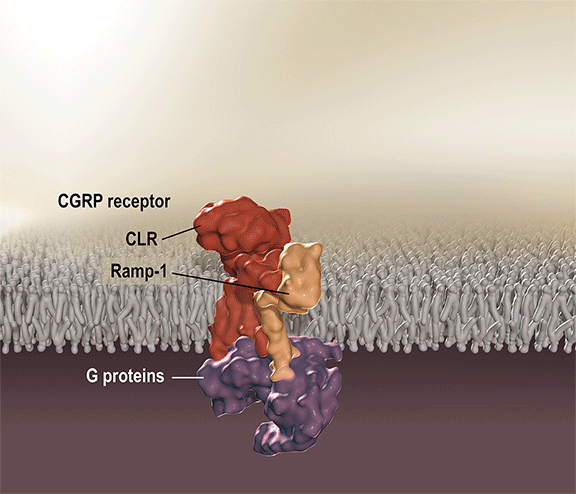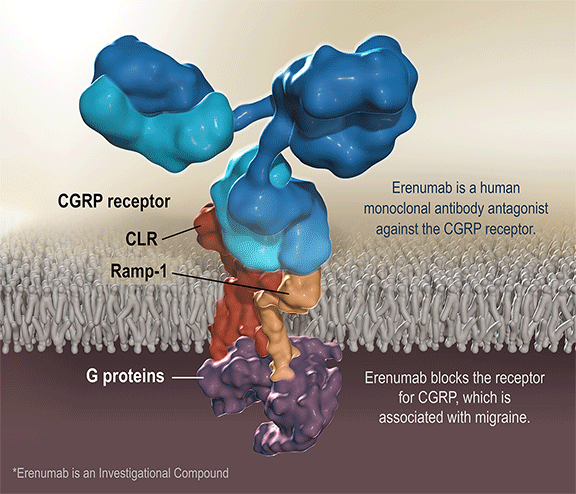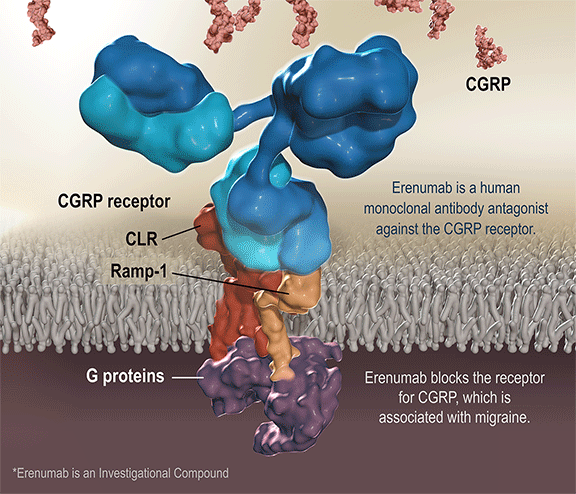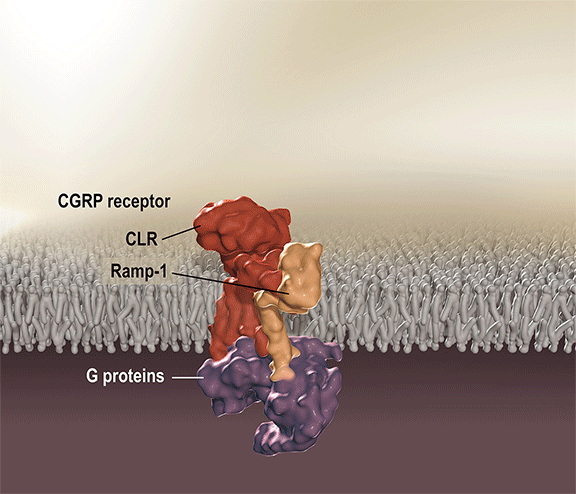
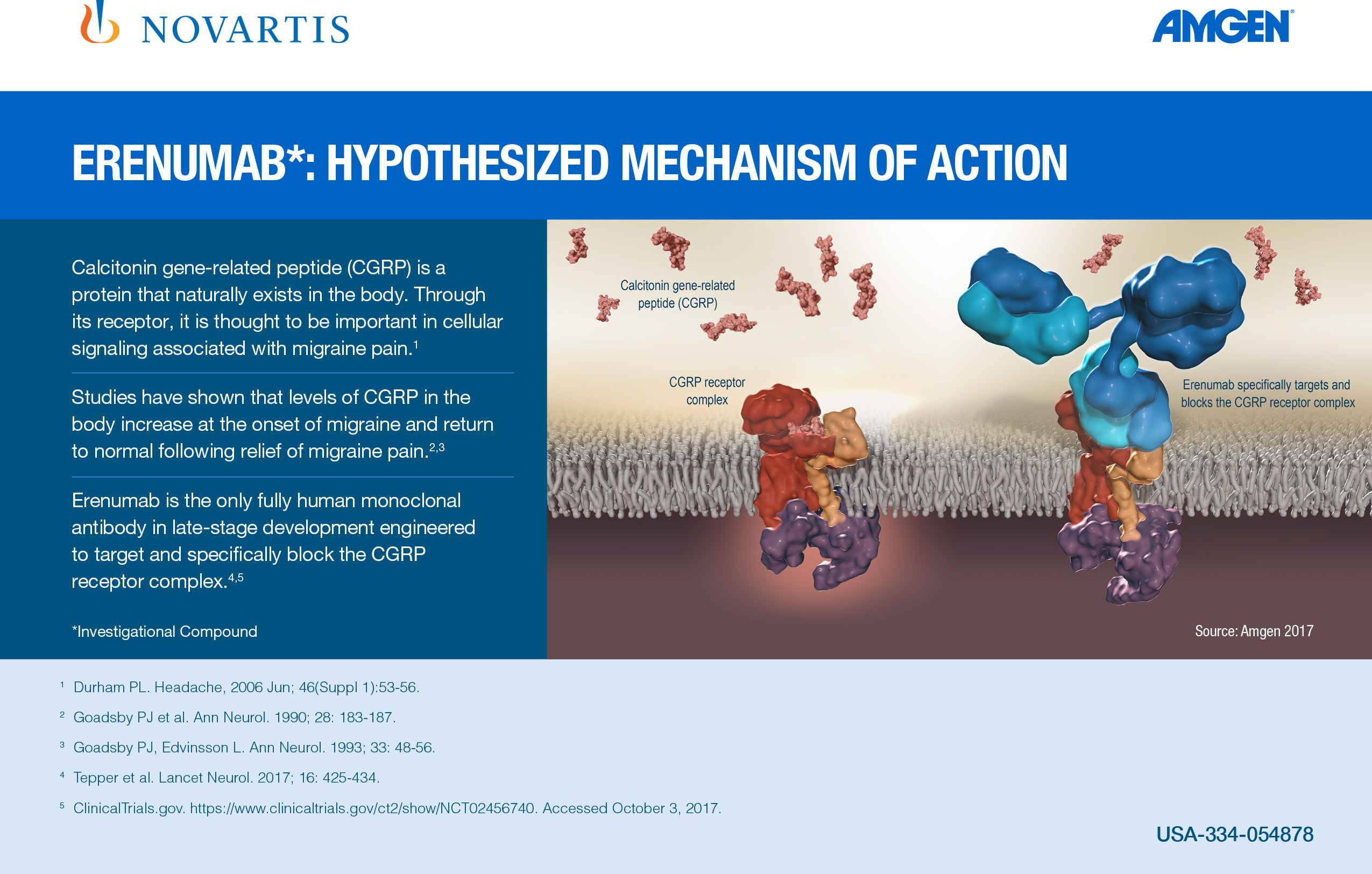
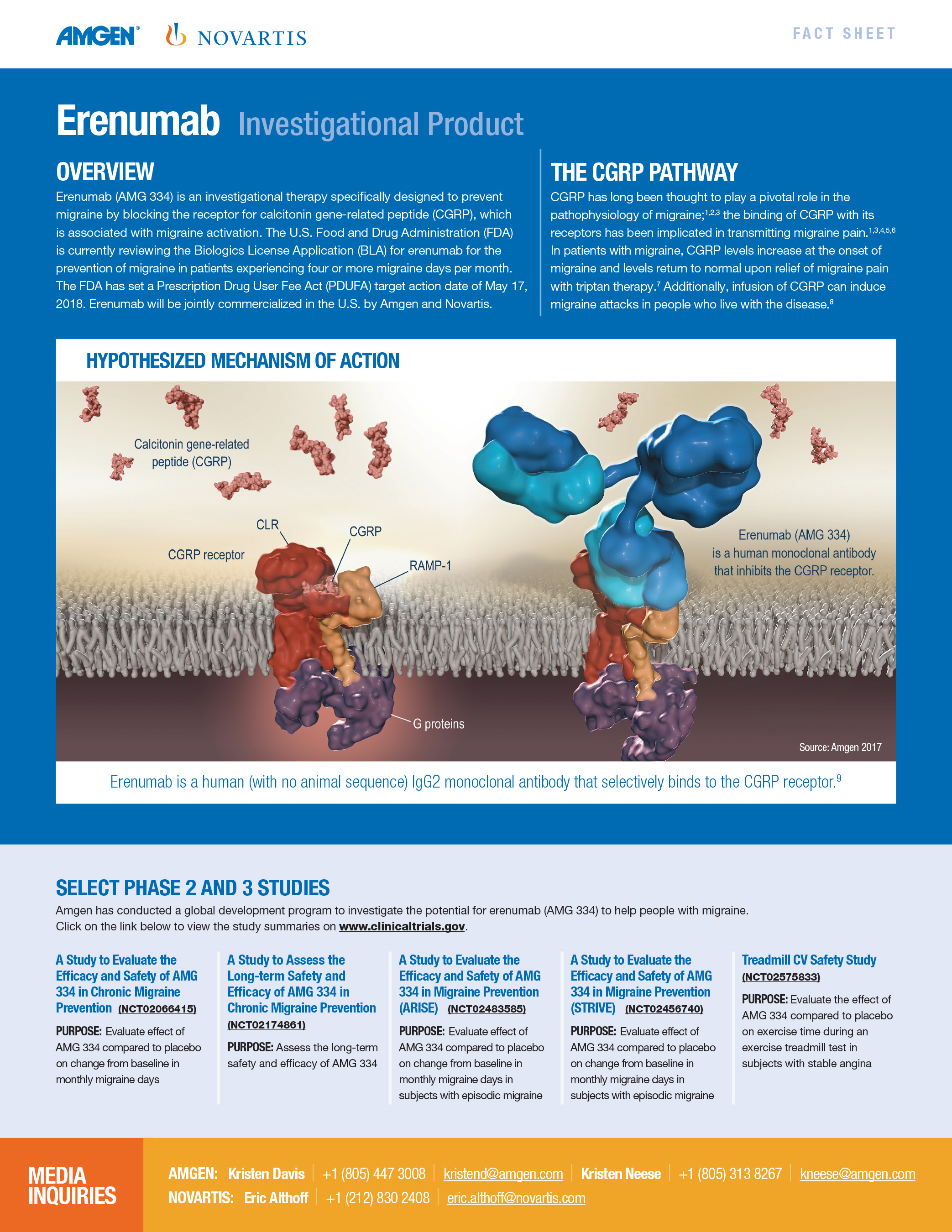
Aimovig is the only treatment specifically designed to prevent migraine by blocking the CGRP receptor, which is associated with migraine activation. Aimovig has been studied in several large global, randomized, double-blind, placebo-controlled studies to assess its safety and efficacy in migraine prevention. More than 2,600 patients have participated in the Aimovig clinical program across the four placebo-controlled Phase 2 and Phase 3 clinical studies and their open-label extensions. Regulatory submissions have been filed in the U.S. and
About Migraine
People with frequent migraine may lose more than half their life to migraine days.3 Migraine robs individuals of time with their families, productivity at home and at work, and their livelihoods. Migraine sufferers endure debilitating pain, physical impairment, and live in constant dread of the next attack – all of which is compounded by a widespread misperception of the disease.4
About
In
About
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen focuses on areas of high unmet medical need and leverages its expertise to strive for solutions that improve health outcomes and dramatically improve people's lives. A biotechnology pioneer since 1980, Amgen has grown to be one of the world's leading independent biotechnology companies, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential.
For more information, visit www.amgen.com and follow us on www.twitter.com/amgen.
Forward-Looking Statements
This news release contains forward-looking statements that are based on the current expectations and beliefs of
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. Even when clinical trials are successful, regulatory authorities may question the sufficiency for approval of the trial endpoints we have selected. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products, including our devices, after they are on the market.
Our results may be affected by our ability to successfully market both new and existing products domestically and internationally, clinical and regulatory developments involving current and future products, sales growth of recently launched products, competition from other products including biosimilars, difficulties or delays in manufacturing our products and global economic conditions. In addition, sales of our products are affected by pricing pressure, political and public scrutiny and reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment. Furthermore, our research, testing, pricing, marketing and other operations are subject to extensive regulation by domestic and foreign government regulatory authorities. Our business may be impacted by government investigations, litigation and product liability claims. In addition, our business may be impacted by the adoption of new tax legislation or exposure to additional tax liabilities. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. Further, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors, or we may fail to prevail in present and future intellectual property litigation. We perform a substantial amount of our commercial manufacturing activities at a few key facilities, including
The scientific information discussed in this news release related to our product candidates is preliminary and investigative. Such product candidates are not approved by the
*The trade name Aimovig™ is provisionally approved for use by the
CONTACT:
References
1 Kawata, et al. Presented at 69th Annual Meeting of the
2 Roberts L, et al. Methods for Addressing Challenges for Evaluating Patient-Reported Outcomes in Clinical Trials of Prophylactic Treatments for Migraines. Presented at 58th Annual Meeting of the
3 Lipton RB, et al. Migraine prevalence, disease burden, and the need for preventative therapy. Neurology. 2007; 68(5):343-9.
4 Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443-54
5 Headache disorders - Fact sheets.
6 Marketscan data on file.
7 Hepp Z et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015; 35(6):478-88.


SOURCE