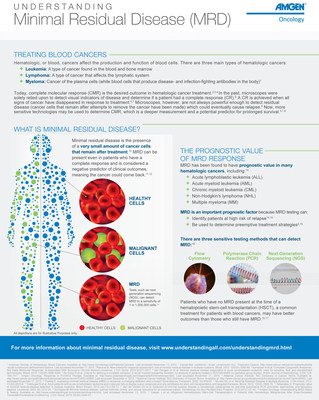
In a Phase 2 confirmatory multicenter single-arm trial (BLAST), adult patients with B-cell precursor ALL with minimal residual disease (MRD) who received BLINCYTO monotherapy demonstrated clinically meaningful relapse-free survival (RFS), as measured in the key secondary endpoint (abstract #680). Median RFS was 18.9 months following initiation of BLINCYTO. MRD refers to the presence of leukemia blast cells below the limits of detection available with standard assessment. Results from the Phase 2 BLAST trial were nominated for inclusion in the Best of ASH Session on
Other presentations demonstrate BLINCYTO's potential in a high risk subpopulation of patients with relapsed or refractory
"A key goal in the treatment of blood cancers is to prevent relapse from occurring," said
ALL is a rare and rapidly progressing cancer of the blood and bone marrow.1,2 In adult patients with relapsed or refractory ALL, median overall survival (OS) is just three to five months.3 Currently, there is no broadly accepted standard treatment regimen for adult patients with relapsed or refractory ALL beyond chemotherapy.4 Around 15-30 percent of adult ALL patients are Ph+ and these patients typically have a poor response to standard therapy, short remission duration and low survival rates.5
Abstracts are currently available on the ASH website.
ASH Abstract #680: Long-Term Outcomes After Blinatumomab Treatment: Follow-up of a Phase 2 Study in Patients With Minimal Residual Disease (MRD) Positive B-cell Precursor ALL
ASH Abstract #679: Complete Molecular and Hematologic Response in Adult Patients with Relapsed/Refractory (R/R) Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia (ALL) Following Treatment with Blinatumomab: Results from a Phase 2 Single-Arm, Multicenter Study (ALCANTARA)
ASH Abstract #861: Treatment with anti-CD19 BiTE® Blinatumomab in Adult Patients with Relapsed/Refractory B-precursor Acute Lymphoblastic Leukemia (r/r ALL) Post-Allogeneic Hematopoietic Stem Cell Transplantation
Amgen Webcast Investor Meeting
Live audio of the conference call will be simultaneously broadcast over the Internet and will be available to members of the news media, investors and the general public.
The webcast, as with other selected presentations regarding developments in
About BLINCYTO® (blinatumomab)
BLINCYTO is a bispecific CD19-directed CD3 T cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells. BLINCYTO was granted breakthrough therapy and priority review designations by the
About BiTE® Technology
BiTE® antibody constructs are a type of immunotherapy being investigated for fighting cancer by helping the body's immune system to detect and target malignant cells. The modified antibodies are designed to engage two different targets simultaneously, thereby juxtaposing T cells (a type of white blood cell capable of killing other cells perceived as threats) to cancer cells. BiTE® antibody constructs help place the T cells within reach of the targeted cell, with the intent of allowing T cells to inject toxins and trigger the cancer cell to die (apoptosis). BiTE® antibody constructs are currently being investigated for their potential to treat a wide variety of cancers.
BLINCYTO® U.S. Product Safety Information
Important Safety Information Regarding BLINCYTO® (blinatumomab) U.S. Indication
This safety information is specific to the current U.S. approved indication.
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES
Contraindications
BLINCYTO® is contraindicated in patients with a known hypersensitivity to blinatumomab or to any component of the product formulation.
Warnings and Precautions
Cytokine Release Syndrome (CRS): Life-threatening or fatal CRS occurred in patients receiving BLINCYTO®. Infusion reactions have occurred and may be clinically indistinguishable from manifestations of CRS. Closely monitor patients for signs and symptoms of serious events such as pyrexia, headache, nausea, asthenia, hypotension, increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased total bilirubin (TBILI), disseminated intravascular coagulation (DIC), capillary leak syndrome (CLS), and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS). Interrupt or discontinue BLINCYTO® as outlined in the Prescribing Information (PI).
Neurological Toxicities: Approximately 50% of patients receiving BLINCYTO® in clinical trials experienced neurological toxicities. Severe, life-threatening, or fatal neurological toxicities occurred in approximately 15% of patients, including encephalopathy, convulsions, speech disorders, disturbances in consciousness, confusion and disorientation, and coordination and balance disorders. The median time to onset of any neurological toxicity was 7 days. Monitor patients for signs or symptoms and interrupt or discontinue BLINCYTO® as outlined in the PI.
Infections: Approximately 25% of patients receiving BLINCYTO® experienced serious infections, some of which were life-threatening or fatal. Administer prophylactic antibiotics and employ surveillance testing as appropriate during treatment. Monitor patients for signs or symptoms of infection and treat appropriately, including interruption or discontinuation of BLINCYTO® as needed.
Tumor Lysis Syndrome (TLS): Life-threatening or fatal TLS has been observed. Preventive measures, including pretreatment nontoxic cytoreduction and on treatment hydration, should be used during BLINCYTO® treatment. Monitor patients for signs and symptoms of TLS and interrupt or discontinue BLINCYTO® as needed to manage these events.
Neutropenia and Febrile Neutropenia, including life-threatening cases, have been observed. Monitor appropriate laboratory parameters during BLINCYTO® infusion and interrupt BLINCYTO® if prolonged neutropenia occurs.
Effects on Ability to Drive and Use Machines: Due to the possibility of neurological events, including seizures, patients receiving BLINCYTO® are at risk for loss of consciousness, and should be advised against driving and engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery while BLINCYTO® is being administered.
Elevated Liver Enzymes: Transient elevations in liver enzymes have been associated with BLINCYTO® treatment. The majority of these events were observed in the setting of CRS. The median time to onset was 15 days. Grade 3 or greater elevations in liver enzymes occurred in 6% of patients outside the setting of CRS and resulted in treatment discontinuation in less than 1% of patients. Monitor ALT, AST, gamma-glutamyl transferase (GGT), and TBILI prior to the start of and during BLINCYTO® treatment. BLINCYTO® treatment should be interrupted if transaminases rise to > 5 times the upper limit of normal (ULN) or if TBILI rises to > 3 times ULN.
Leukoencephalopathy: Although the clinical significance is unknown, cranial magnetic resonance imaging (MRI) changes showing leukoencephalopathy have been observed in patients receiving BLINCYTO®, especially in patients previously treated with cranial irradiation and anti-leukemic chemotherapy. Preparation and administration errors have occurred with BLINCYTO® treatment. Follow instructions for preparation (including admixing) and administration in the PI strictly to minimize medication errors (including underdose and overdose).
Adverse Reactions
The most commonly reported adverse reactions (≥ 20%) in clinical trials were pyrexia (62%), headache (36%), peripheral edema (25%), febrile neutropenia (25%), nausea (25%), hypokalemia (23%), rash (21%), tremor (20%), diarrhea (20%) and constipation (20%).
Serious adverse reactions were reported in 65% of patients. The most common serious adverse reactions (≥ 2%) included febrile neutropenia, pyrexia, pneumonia, sepsis, neutropenia, device-related infection, tremor, encephalopathy, infection, overdose, confusion, Staphylococcal bacteremia, and headache.
U.S. Dosage and Administration Guidelines
BLINCYTO® is administered as a continuous intravenous infusion at a constant flow rate using an infusion pump which should be programmable, lockable, non-elastomeric, and have an alarm. It is very important that the instructions for preparation (including admixing) and administration provided in the full Prescribing Information are strictly followed to minimize medication errors (including underdose and overdose).
Please see full U.S. Prescribing Information and medication guide for BLINCYTO® at www.BLINCYTO.com.
About Amgen
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen focuses on areas of high unmet medical need and leverages its biologics manufacturing expertise to strive for solutions that improve health outcomes and dramatically improve people's lives. A biotechnology pioneer since 1980, Amgen has grown to be one of the world's leading independent biotechnology companies, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential.
For more information, visit www.amgen.com and follow us on www.twitter.com/amgen.
About
Amgen Oncology is committed to helping patients take on some of the toughest cancers, such as those that have been resistant to drugs, those that progress rapidly through the body and those where limited treatment options exist.
Forward-Looking Statements
This news release contains forward-looking statements that are based on the current expectations and beliefs of
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us and our partners to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products after they are on the market. Our business may be impacted by government investigations, litigation and product liability claims. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. We depend on third parties for a significant portion of our manufacturing capacity for the supply of certain of our current and future products and limits on supply may constrain sales of certain of our current products and product candidate development.
In addition, sales of our products (including products of our wholly-owned subsidiaries) are affected by the reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment as well as U.S. legislation affecting pharmaceutical pricing and reimbursement. Government and others' regulations and reimbursement policies may affect the development, usage and pricing of our products. In addition, we compete with other companies with respect to some of our marketed products as well as for the discovery and development of new products. We believe that some of our newer products, product candidates or new indications for existing products, may face competition when and as they are approved and marketed. Our products may compete against products that have lower prices, established reimbursement, superior performance, are easier to administer, or that are otherwise competitive with our products. In addition, while
The scientific information discussed in this news release relating to new indications for
CONTACT:
References:

Photo - http://photos.prnewswire.com/prnh/20151207/293533-INFO
Logo - http://photos.prnewswire.com/prnh/20081015/AMGENLOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/amgen-presents-data-from-three-trials-evaluating-blincyto-blinatumomab-in-acute-lymphoblastic-leukemia-at-ash-2015-300188679.html
SOURCE