Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8490257-amgen-nature-amg510-publication/
Titled "The Clinical KRASG12C Inhibitor AMG 510 Drives Anti-Tumor Immunity," the paper highlights novel structural insights that led to the discovery of AMG 510, the preclinical evidence of AMG 510 activity, its potential ability to induce tumor-cell killing as both a monotherapy and in combination with other therapies, and its impact on the immune system that may render tumor cells particularly sensitive to immunotherapy. Early evidence of clinical activity of AMG 510 is also presented in the paper.
"We are pleased to share how our team of scientists at
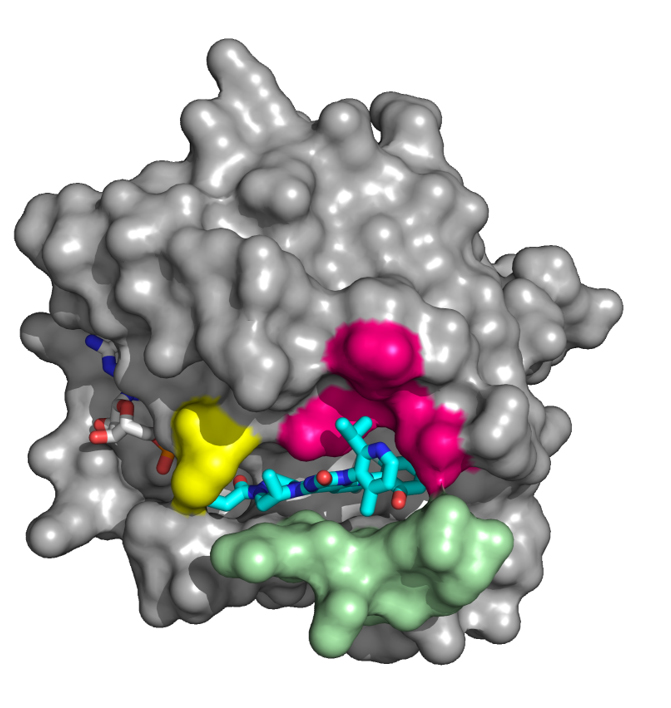
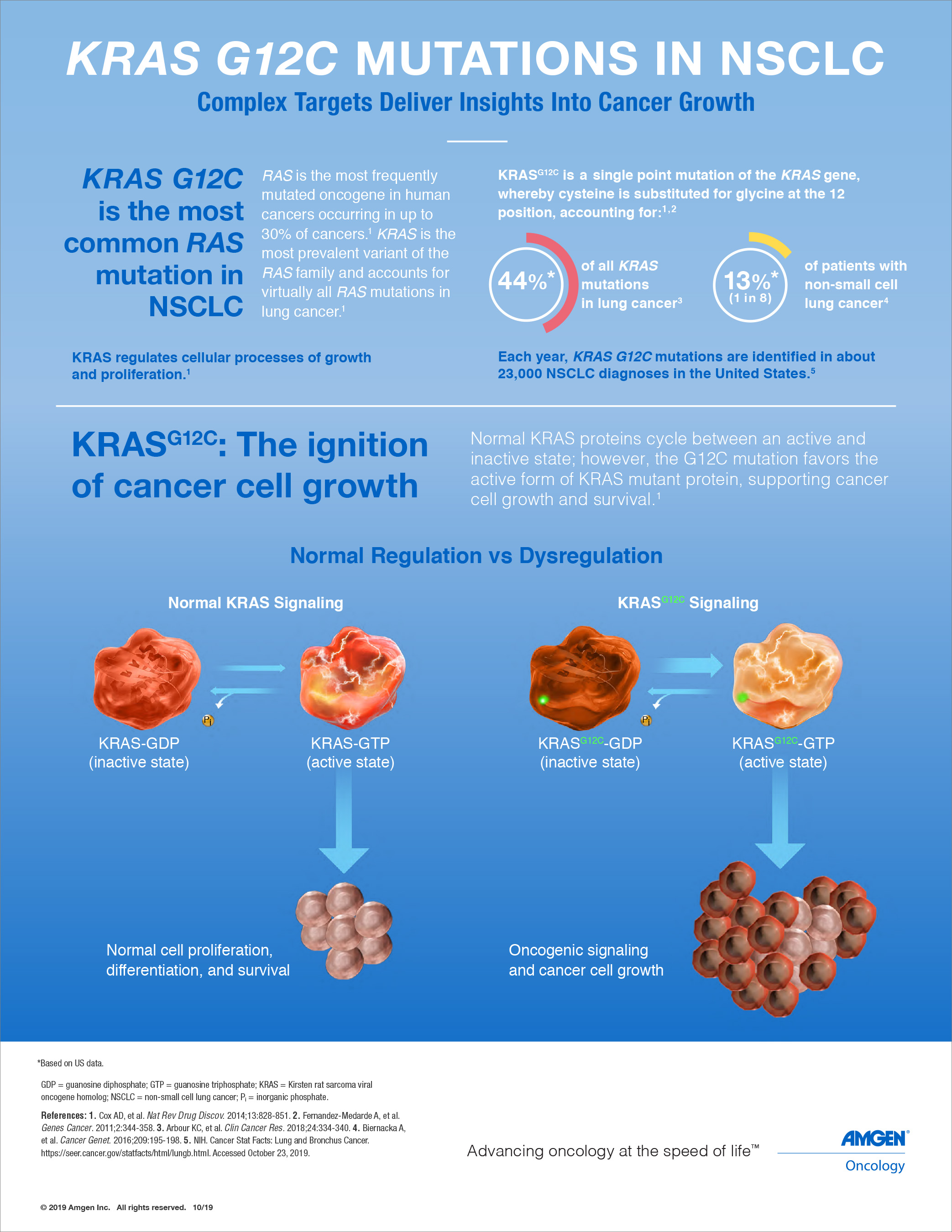
KRAS, identified over 30 years ago as a proto-oncogene, is one of the most frequently mutated oncogenes in human cancer.1,2 Amgen researchers first identified the novel histidine 95 (H95) groove located on an inactive KRASG12C protein. Through extensive compound screening and structure-based design, AMG 510 emerged as the top investigational candidate from the optimization of a series of H95 groove-binding molecules. It is designed to irreversibly bind to KRASG12C protein and permanently lock it in an inactive state, leading to inhibition of tumor cell growth in KRASG12C driven tumors. In preclinical experiments, AMG 510 demonstrated favorable potency and selectivity, and induced regression in mice bearing KRASG12C mutated tumors.
"There is a significant unmet need for tumor-selective therapies that minimize a negative impact on normal cells, and many patients diagnosed with KRAS-mutated solid tumors have typically faced a challenging prognosis with limited targeted treatment options," said
About KRAS
The subject of more than three decades of research, the RAS gene family are the most frequently mutated oncogenes in human cancers.1,2 Within this family, KRAS is the most prevalent variant and is particularly common in solid tumors.2 A specific mutation known as KRAS G12C accounts for approximately 13% of non-small cell lung cancers, three to five percent of colorectal cancers and one to two percent of numerous other solid tumors.3 Approximately 30,000 patients are diagnosed each year in
About Amgen Oncology
Amgen Oncology is searching for and finding answers to incredibly complex questions that will advance care and improve lives for cancer patients and their families. Our research drives us to understand the disease in the context of the patient's life – not just their cancer journey – so they can take control of their lives.
For the last four decades, we have been dedicated to discovering the firsts that matter in oncology and to finding ways to reduce the burden of cancer. Building on our heritage,
At
For more information, follow us on www.twitter.com/amgenoncology.
About
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen focuses on areas of high unmet medical need and leverages its expertise to strive for solutions that improve health outcomes and dramatically improve people's lives. A biotechnology pioneer since 1980, Amgen has grown to be one of the world's leading independent biotechnology companies, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential.
For more information, visit www.amgen.com and follow us on www.twitter.com/amgen.
Forward-Looking Statements
This news release contains forward-looking statements that are based on the current expectations and beliefs of
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. Even when clinical trials are successful, regulatory authorities may question the sufficiency for approval of the trial endpoints we have selected. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products, including our devices, after they are on the market.
Our results may be affected by our ability to successfully market both new and existing products domestically and internationally, clinical and regulatory developments involving current and future products, sales growth of recently launched products, competition from other products including biosimilars, difficulties or delays in manufacturing our products and global economic conditions. In addition, sales of our products are affected by pricing pressure, political and public scrutiny and reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment. Furthermore, our research, testing, pricing, marketing and other operations are subject to extensive regulation by domestic and foreign government regulatory authorities. Our business may be impacted by government investigations, litigation and product liability claims. In addition, our business may be impacted by the adoption of new tax legislation or exposure to additional tax liabilities. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. Further, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors, or we may fail to prevail in present and future intellectual property litigation. We perform a substantial amount of our commercial manufacturing activities at a few key facilities, including in
The scientific information discussed in this news release related to
CONTACT:

![]() View original content:http://www.prnewswire.com/news-releases/the-discovery-of-amgens-novel-investigational-krasg12c-inhibitor-amg-510-published-in-nature-300948664.html
View original content:http://www.prnewswire.com/news-releases/the-discovery-of-amgens-novel-investigational-krasg12c-inhibitor-amg-510-published-in-nature-300948664.html
SOURCE