One of the most common side effects of myelosuppressive chemotherapy is a low white blood cell count, or neutropenia. Febrile neutropenia (neutropenia with fever) is a medical emergency and is associated with an increased risk of hospitalization and use of IV anti-infective drugs. Among all cancer patients, including those who did or did not receive a granulocyte-colony stimulating factor (G-CSF), neutropenia and infectious complications resulted in an estimated 200,000 to 330,000 hospitalizations in 2009 in the U.S.3-7
Although Neulasta has been available for 12 years, some patients still do not receive their Neulasta at least 24 hours after cytotoxic chemotherapy as specified in the Neulasta PI.2 Among appropriate patients receiving myelosuppressive chemotherapy, many return to their HCP one day after chemotherapy treatment for the sole purpose of receiving a Neulasta injection; however, a portion of patients requiring Neulasta may not be able to return to their HCP, which means they may not be in accordance with PI recommended dosing. With the Neulasta Delivery Kit, HCPs now have an administration option for patients who would not need to return to the clinic or hospital the day after chemotherapy for anything other than their Neulasta injection.
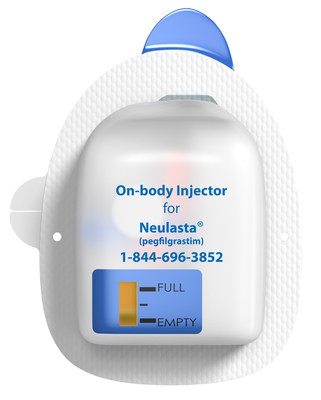
Results from a Phase 1 pharmacokinetic study demonstrated that the On-body Injector for Neulasta offers comparable pharmacokinetics to Neulasta delivered via the prefilled syringe for manual use.2
In addition to the On-body Injector, the Neulasta Delivery Kit includes a specifically designed Neulasta prefilled syringe along with HCP and Patient Instructions for Use and a Quick Reference Guide. On the same day as a chemotherapy session, the HCP initiates Neulasta administration by using the co-packaged syringe to fill the injector and activate it. The On-body Injector is then applied to the patient, to deliver Neulasta approximately 27 hours after the administration of cytotoxic chemotherapy. Activation of the injector leads to the subsequent insertion of the subcutaneous cannula while under HCP supervision. The HCP then reviews and provides the patient with the Patient Instructions for Use, which includes information about the On-body Injector for Neulasta and what the patient can expect while wearing it.
About Febrile Neutropenia
One of the most common side effects of myelosuppressive chemotherapy is a low white blood cell count. An abnormally low level of neutrophils, important infection-fighting white blood cells, is called neutropenia. The fewer neutrophils a patient has – and the longer the neutrophil count remains low – the greater the risk of developing a potentially serious infection.8
Febrile neutropenia is neutropenia complicated by a fever. Fever is frequently a sign of infection and, in patients receiving myelosuppressive chemotherapy, it can sometimes be the only sign. Febrile neutropenia is a medical emergency and is associated with several potential downstream consequences.
About Neulasta
Neulasta is a leukocyte growth factor approved by the
In a pivotal clinical trial, in patients with nonmyeloid malignancies undergoing myelosuppressive chemotherapy associated with a clinically significant incidence of febrile neutropenia, treatment with Neulasta has been shown to significantly reduce the incidence of febrile neutropenia as well as hospitalizations related to febrile neutropenia and the use of IV antibiotics.9
For more information about Neulasta, visit www.Neulasta.com and www.NeulastaHCP.com.
Important Safety Information
Do not administer Neulasta to patients with a history of serious allergic reactions to pegfilgrastim or filgrastim.
Fatal splenic rupture can occur. Evaluate for splenomegaly or splenic rupture in patients with left upper abdominal or shoulder pain. Acute respiratory distress syndrome (ARDS) can occur. Evaluate for ARDS in patients who develop fever, lung infiltrates, or respiratory distress. Discontinue Neulasta in patients with ARDS. Serious allergic reactions, including anaphylaxis, can occur. Permanently discontinue Neulasta in patients with serious allergic reactions. The On-body Injector for Neulasta uses acrylic adhesive. For patients who have reactions to acrylic adhesives, use of this product may result in a significant reaction. Severe and sometimes fatal sickle cell crises have been reported.
Most common adverse reactions (≥ 5 percent difference in incidence) in placebo-controlled clinical trials are bone pain and pain in extremity.
Please see additional Neulasta Safety Information, by visiting
www.amgen.com/medpro/products.html.
Please see the Neulasta Full Prescribing Information by clicking here http://pi.amgen.com/united_states/neulasta/neulasta_pi_hcp_english.pdf.
About
For more information, visit www.amgen.com and follow us on www.twitter.com/amgen.
Forward-Looking Statements
This news release contains forward-looking statements that are based on the current expectations and beliefs of
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us and our partners to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products after they are on the market. Our business may be impacted by government investigations, litigation and product liability claims. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. We depend on third parties for a significant portion of our manufacturing capacity for the supply of certain of our current and future products and limits on supply may constrain sales of certain of our current products and product candidate development.
In addition, sales of our products (including products of our wholly-owned subsidiaries) are affected by the reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment as well as U.S. legislation affecting pharmaceutical pricing and reimbursement. Government and others' regulations and reimbursement policies may affect the development, usage and pricing of our products. In addition, we compete with other companies with respect to some of our marketed products as well as for the discovery and development of new products. We believe that some of our newer products, product candidates or new indications for existing products, may face competition when and as they are approved and marketed. Our products may compete against products that have lower prices, established reimbursement, superior performance, are easier to administer, or that are otherwise competitive with our products. In addition, while we and our partners routinely obtain patents for our and their products and technology, the protection of our products offered by patents and patent applications may be challenged, invalidated or circumvented by our or our partners' competitors and there can be no guarantee of our or our partners' ability to obtain or maintain patent protection for our products or product candidates. We cannot guarantee that we will be able to produce commercially successful products or maintain the commercial success of our existing products. Our stock price may be affected by actual or perceived market opportunity, competitive position, and success or failure of our products or product candidates. Further, the discovery of significant problems with a product similar to one of our products that implicate an entire class of products could have a material adverse effect on sales of the affected products and on our business and results of operations. Our efforts to integrate the operations of companies we have acquired may not be successful. Cost savings initiatives may result in us incurring impairment or other related charges on our assets. We may experience difficulties, delays or unexpected costs and not achieve anticipated benefits and savings from our recently announced restructuring plan. Our business performance could affect or limit the ability of our Board of Directors to declare a dividend or our ability to pay a dividend or repurchase common stock.
CONTACT:
|
References |
|
|
1. |
Neulasta® (pegfilgrastim) Delivery Kit healthcare provider instructions for use. Thousand Oaks, California: Amgen (2014). |
|
2. |
Neulasta® (pegfilgrastim) prescribing information. Thousand Oaks, California: Amgen (2002). |
|
3. |
Amgen Data on File. |
|
4. |
Dictionary of Cancer Terms: Febrile Neutropenia. National Cancer Institute website. Available at www.cancer.gov/dictionary?CdrID=415543. Accessed October 31, 2013. |
|
5. |
Kuderer N, et al. Cancer. 2006: 2006;106:2258–66. |
|
6. |
NCI "Chemotherapy and You" pamphlet. National Cancer Institute website. Available at www.cancer.gov/cancertopics/coping/chemotherapy-and-you.pdf. Accessed October 31, 2013. |
|
7. |
Anhang Price R et al. Cancer Hospitalizations for Adults. HCUP. 2009: Table 5. |
|
8. |
Bodey G, et al. Ann Intern Med. 1966;64(2); 328-340. |
|
9. |
Vogel C, et al. J Clin Oncol. 2005; 22: 1178-1184 |
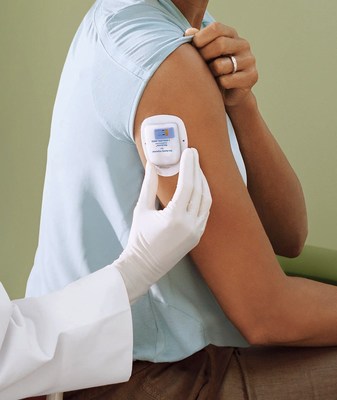

Photo - http://photos.prnewswire.com/prnh/20150302/178857
Photo - http://photos.prnewswire.com/prnh/20150302/178867
Logo - http://photos.prnewswire.com/prnh/20081015/AMGENLOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/amgen-announces-launch-of-new-neulasta-pegfilgrastim-delivery-kit-300043818.html
SOURCE