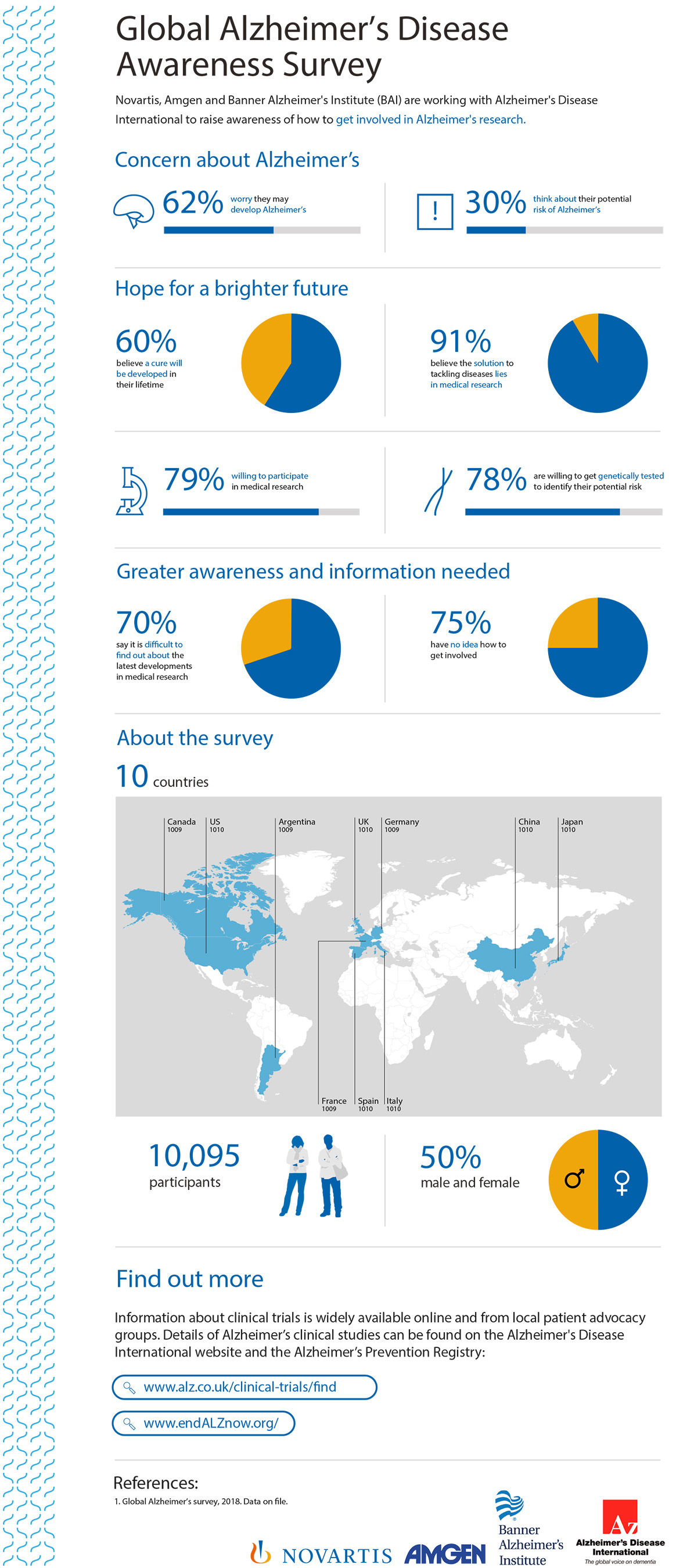
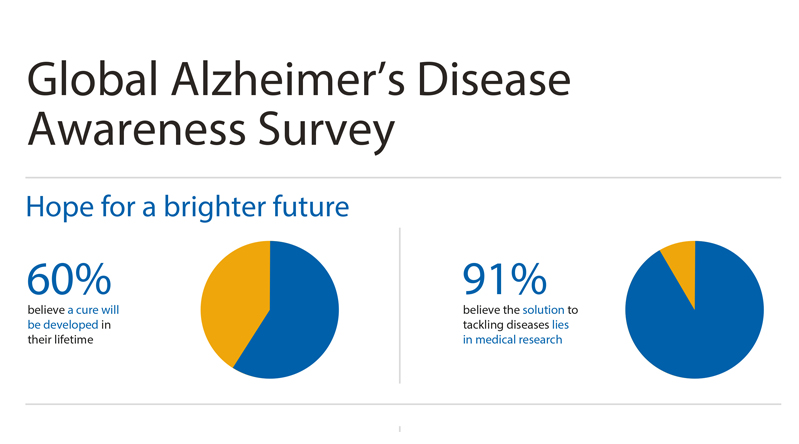
THOUSAND OAKS, Calif., Sept. 17, 2018 /PRNewswire/ -- Amgen (NASDAQ:AMGN), Novartis and Banner Alzheimer's Institute (BAI), in association with Alzheimer's Disease International (ADI), today announced results from the largest global survey to date investigating perceptions of Alzheimer's disease. Findings show that most adults (62 percent) are worried they may develop Alzheimer's, while nearly the same proportion believe it is likely a cure will be developed in their lifetime (60 percent).1 This World Alzheimer's Month, Amgen, Novartis, BAI and ADI are raising awareness about how volunteers can take part in clinical studies to benefit Alzheimer's research, potentially themselves and future generations.
To view the Multimedia News Release, go to: https://www.multivu.com/players/English/8004557-amgen-global-alzheimers-disease-awareness-survey/.
Alzheimer's is the leading cause of dementia, a disease affecting 50 million people worldwide and projected to triple by 2050.2 The survey of more than 10,000 people across 10 countries revealed that 91 percent believe the solution to tackling diseases lies in medical research, and 79 percent are willing to participate.1 However, three-quarters (75 percent) have no idea how to get involved in medical research.1 In addition, 78 percent of adults are willing to get genetically tested to identify their potential risk of developing Alzheimer's.1
"At present, there is no cure and limited treatment options for Alzheimer's, but this survey clearly shows that people are willing to participate in research to help treat and to hopefully find a cure," said Paola Barbarino, chief executive officer at ADI. "We need to demystify and remove awareness barriers to participation in medical research, making all suitable candidates aware of how they can get involved."
Worldwide, more than 400 clinical studies are recruiting in Alzheimer's.3 However, slow enrollment is a costly and common obstacle that undermines medical research.4 There is a need for more people to volunteer to advance scientific discovery.
"The results from this survey clearly demonstrate the need to raise awareness about clinical studies globally," said Pierre N. Tariot, M.D., director of BAI and co-director of the Alzheimer's Prevention Initiative (API). "Aside from funding, the greatest challenge in finding a way to treat, slow, or prevent Alzheimer's is the recruitment and retention of study participants. Scientists are making great progress in the fight against this disease, but an estimated 80 percent of studies fail to meet recruitment goals on time, which delays critically important research."
September 2018 marks the 7th World Alzheimer's Month and represents a chance for people to raise awareness, fundraise and find out more about how they can participate in research. In addition to the global survey results, ADI will also launch their World Alzheimer Report 2018 entitled The state of the art of dementia research: new frontiers, which looks at the hopes and aspirations, the barriers and enablers to improving dementia research globally. More information on the report can be found here https://www.alz.co.uk/worldreport2018.
"Amgen, along with Novartis, Banner Alzheimer's Institute and Alzheimer's Disease International, is committed to raising awareness of how to enroll in Alzheimer's research to empower patients and healthy volunteers," said David M. Reese, M.D., executive vice president of Research and Development at Amgen. "Alzheimer's research often does not get the attention and support it deserves. These survey results illustrate the significant public worry about developing Alzheimer's and reinforce the need to raise greater awareness of how people can participate in clinical studies to contribute to Alzheimer's research."
Amgen is currently enrolling volunteers for clinical trials in Alzheimer's research. Additional information about clinical trials is widely available online and from local patient advocacy groups. Details of Alzheimer's clinical studies can be found on the ADI website www.alz.co.uk/clinical-trials/find and the Alzheimer's Prevention Registry www.endALZnow.org/. Studies can also be found within the ClinicalTrials.gov study database, https://clinicaltrials.gov, under the search criteria 'Recruiting' and 'Alzheimer Disease'.
About the Survey
The survey was conducted online by The Harris Poll on behalf of Amgen, Novartis and BAI, among 10,095 adults 18+ living in Argentina, Canada, China, France, Germany, Italy, Japan, Spain, UK and the U.S. The survey was conducted between July 25 and Aug. 21, 2018. Figures for age by gender, income, education, race/ethnicity (Canada and U.S. only), region, size of household, marital status, and employment status were weighted where necessary to bring them into line with their actual proportions in the population.
The API is an international collaborative research effort formed to launch a new era of Alzheimer's prevention research. Led by the BAI, the API conducts prevention trials in cognitively healthy people at increased genetic risk for Alzheimer's disease. It will continue to establish the brain imaging, biological and cognitive measurements needed to rapidly test promising prevention therapies and provide registries to support enrollment in future prevention trials. API is intended to provide the scientific means, accelerated approval pathway with the cooperation of the regulatory agencies and enrollment resources needed to evaluate the range of promising Alzheimer's prevention therapies and find ones that work. For more information, go to www.alzheimerspreventioninitiative.com
About the Amgen and Novartis Neuroscience Collaboration
In August 2015, Novartis entered into a global collaboration with Amgen to develop and commercialize pioneering treatments in the field of migraine and Alzheimer's disease.
About Amgen
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen focuses on areas of high unmet medical need and leverages its expertise to strive for solutions that improve health outcomes and dramatically improve people's lives. A biotechnology pioneer since 1980, Amgen has grown to be one of the world's leading independent biotechnology companies, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential.
For more information, visit www.amgen.com and follow us on www.twitter.com/amgen.
Forward-Looking Statements
This news release contains forward-looking statements that are based on the current expectations and beliefs of Amgen. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial metrics, expected legal, arbitration, political, regulatory or clinical results or practices, customer and prescriber patterns or practices, reimbursement activities and outcomes and other such estimates and results. Forward-looking statements involve significant risks and uncertainties, including those discussed below and more fully described in the Securities and Exchange Commission reports filed by Amgen, including our most recent annual report on Form 10-K and any subsequent periodic reports on Form 10-Q and current reports on Form 8-K. Unless otherwise noted, Amgen is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. Even when clinical trials are successful, regulatory authorities may question the sufficiency for approval of the trial endpoints we have selected. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products, including our devices, after they are on the market.
Our results may be affected by our ability to successfully market both new and existing products domestically and internationally, clinical and regulatory developments involving current and future products, sales growth of recently launched products, competition from other products including biosimilars, difficulties or delays in manufacturing our products and global economic conditions. In addition, sales of our products are affected by pricing pressure, political and public scrutiny and reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment. Furthermore, our research, testing, pricing, marketing and other operations are subject to extensive regulation by domestic and foreign government regulatory authorities. Our business may be impacted by government investigations, litigation and product liability claims. In addition, our business may be impacted by the adoption of new tax legislation or exposure to additional tax liabilities. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. Further, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors, or we may fail to prevail in present and future intellectual property litigation. We perform a substantial amount of our commercial manufacturing activities at a few key facilities, including in Puerto Rico, and also depend on third parties for a portion of our manufacturing activities, and limits on supply may constrain sales of certain of our current products and product candidate development. In addition, we compete with other companies with respect to many of our marketed products as well as for the discovery and development of new products. Further, some raw materials, medical devices and component parts for our products are supplied by sole third-party suppliers. Certain of our distributors, customers and payers have substantial purchasing leverage in their dealings with us. The discovery of significant problems with a product similar to one of our products that implicate an entire class of products could have a material adverse effect on sales of the affected products and on our business and results of operations. Our efforts to acquire other companies or products and to integrate the operations of companies we have acquired may not be successful. A breakdown, cyberattack or information security breach could compromise the confidentiality, integrity and availability of our systems and our data security. We are increasingly dependent on information technology systems, infrastructure and data security. Our stock price is volatile and may be affected by a number of events. Our business performance could affect or limit the ability of our Board of Directors to declare a dividend or our ability to pay a dividend or repurchase our common stock. We may not be able to access the capital and credit markets on terms that are favorable to us, or at all.
CONTACT: Amgen, Thousand Oaks
Andrea Fassacesia, 805-905-2575 (Media)
Kristen Neese, 805-313-8267 (Media)
Arvind Sood, 805-447-1060 (Investors)
References
1. Data on file. August 2018.
2. Dementia fact sheet December 2017; World Health Organisation: http://www.who.int/news-room/fact-sheets/detail/dementia Accessed August 2018.
3. ClinicalTrials.gov: //clinicaltrials.gov/ct2/results?recrs=ab&cond=Alzheimer+Disease&term=&cntry=&state=&city=&dist=. Accessed August 2018.
4. Clin Transl Sci. 2015 Dec; 8(6): 647–654.
 View original content:http://www.prnewswire.com/news-releases/worlds-largest-alzheimers-survey-reveals-most-adults-believe-a-cure-will-be-developed-in-their-lifetime-300713961.html
View original content:http://www.prnewswire.com/news-releases/worlds-largest-alzheimers-survey-reveals-most-adults-believe-a-cure-will-be-developed-in-their-lifetime-300713961.html
SOURCE Amgen



![]() View original content:http://www.prnewswire.com/news-releases/worlds-largest-alzheimers-survey-reveals-most-adults-believe-a-cure-will-be-developed-in-their-lifetime-300713961.html
View original content:http://www.prnewswire.com/news-releases/worlds-largest-alzheimers-survey-reveals-most-adults-believe-a-cure-will-be-developed-in-their-lifetime-300713961.html